"A spray, which consisted of very small drops, was found to be quite satisfactory"
"Refrigerated Wind Tunnel Tests on Surface Coatings for Preventing Ice Formation"
Summary
An early icing wind tunnel and test are described
Key Points
- An early icing wind tunnel with most of the components of current tunnels is described.
- A test of several coatings to prevent ice is detailed.
- Many of the points noted about icing tunnel test reflect current test experience.
Abstract
This investigation was conducted in the Refrigerated Wind Tunnel at Langley Memorial Aeronautical Laboratory, Langley Field, Virginia, to determine the effectiveness of various surface coatings as a means for preventing ice formations on aircraft in flight. The substances used as coatings for these tests are divided into two groups: compounds soluble in water, and those which are insoluble in water. It was found that certain soluble compounds were apparently effective in preventing the formation of ice on an airfoil model, while all insoluble compounds which were tested were found to be ineffective.
Discussion
This is a good introduction to icing wind tunnels, as it illustrates the basic components common to most current icing wind tunnels, and it also illustrates some test challenges.
The scale was limited (6-inch diameter throat), which limited the size of the test article (a 3-inch chord Clark Y airfoil construct of mahogany wood).
This review will only superficially cover the test purpose, determining the performance of potential surface coatings to prevent ice formation.
Apparatus
A diagram of the refrigerated wind tunnel, which is of the open-throat type with an air stream 6 inches in diameter, is shown in Figure 1. The tunnel proper, which is of metal, is insulated from the outside air by layers of cork and wood. The test chamber is insulated by double glass doors and windows which enable the ice-forming process to be observed and photographed from the outside (Fig. 2). The air temperature in the tunnel is lowered and regulated by the flow of cold brine through the hollow, metal guide vanes, the brine being cooled by a refrigerating apparatus of commercial type (Fig. 3). The circulation of the air in the tunnel is maintained by a propeller driven by an electric motor.
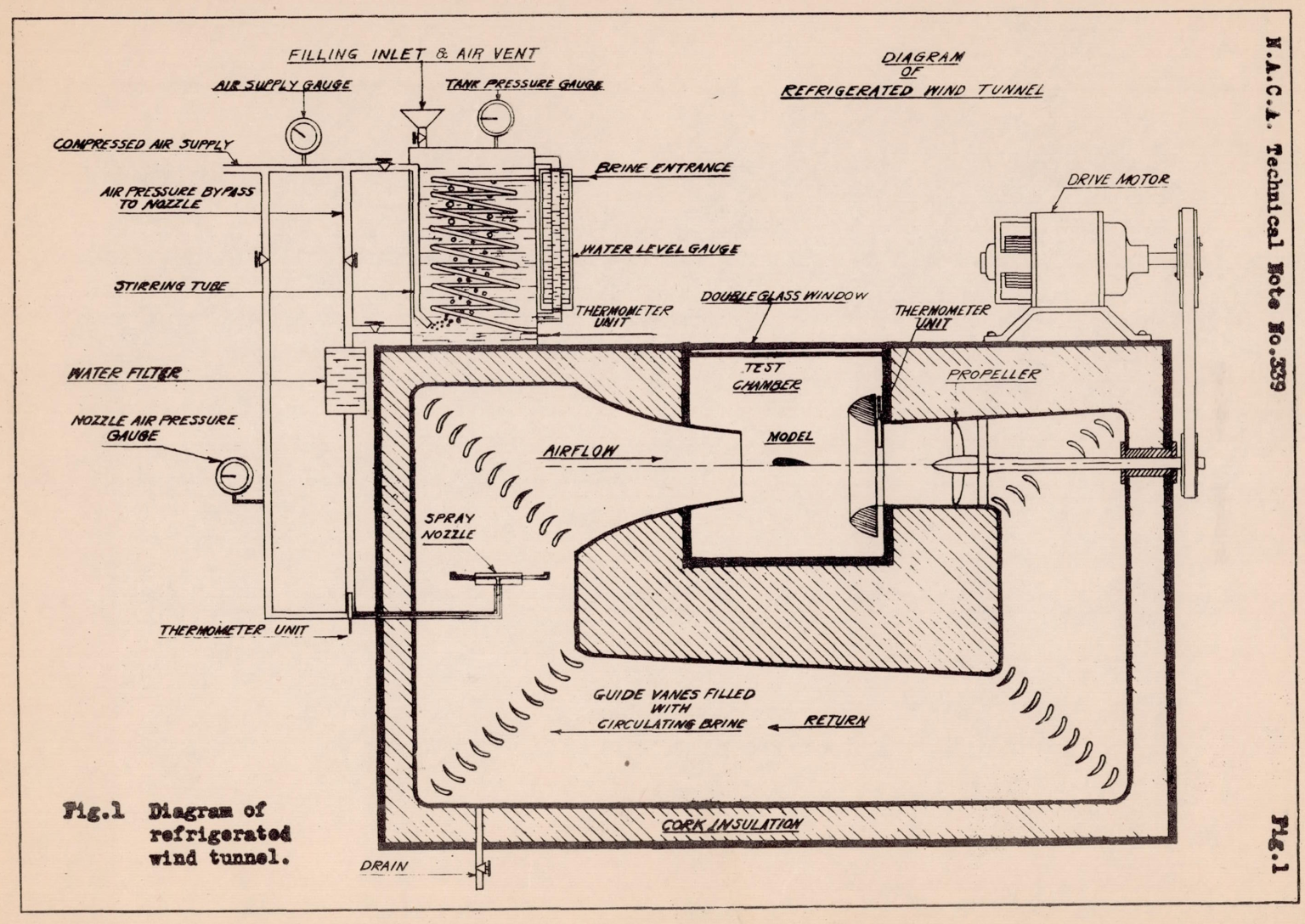
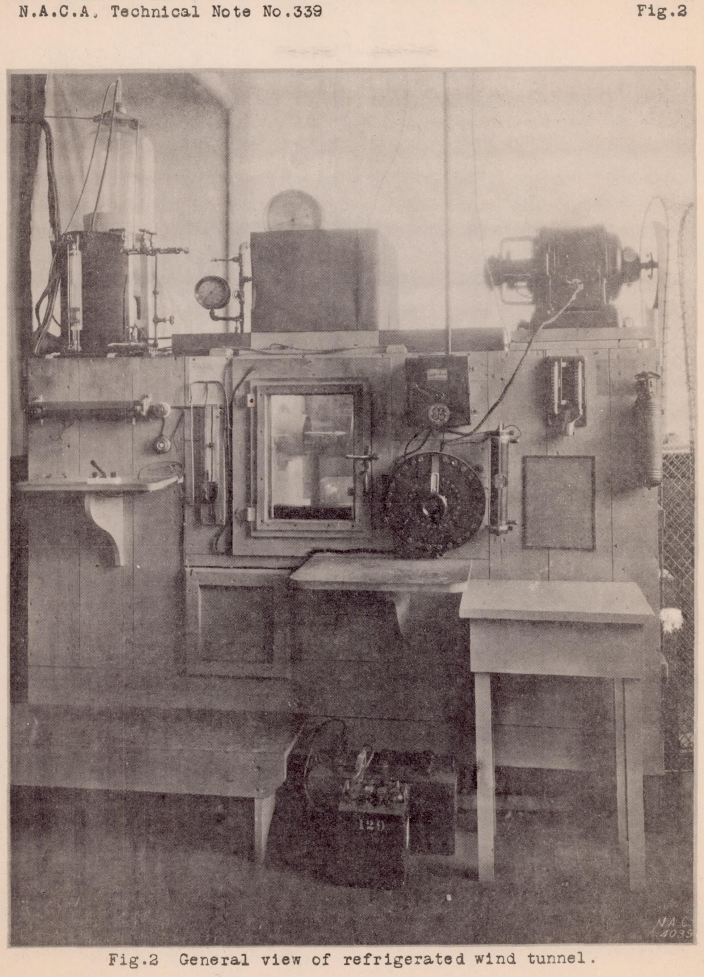
Most current recirculating-loop icing wind tunnels have the same components: fan, turning vanes, converging and diverging sections, refrigeration and a heat exchanger, spray nozzles, and a nominal test section.
There was an array of four water spray nozzles. The basic spray nozzle design is similar to, but not identical to, those used in the later NACA Lewis Icing Research Tunnel (IRT). A central stream of water is affected by circumferential air jets. Adjusting the water pressure and air pressure result in different combinations of drop size and liquid water content.
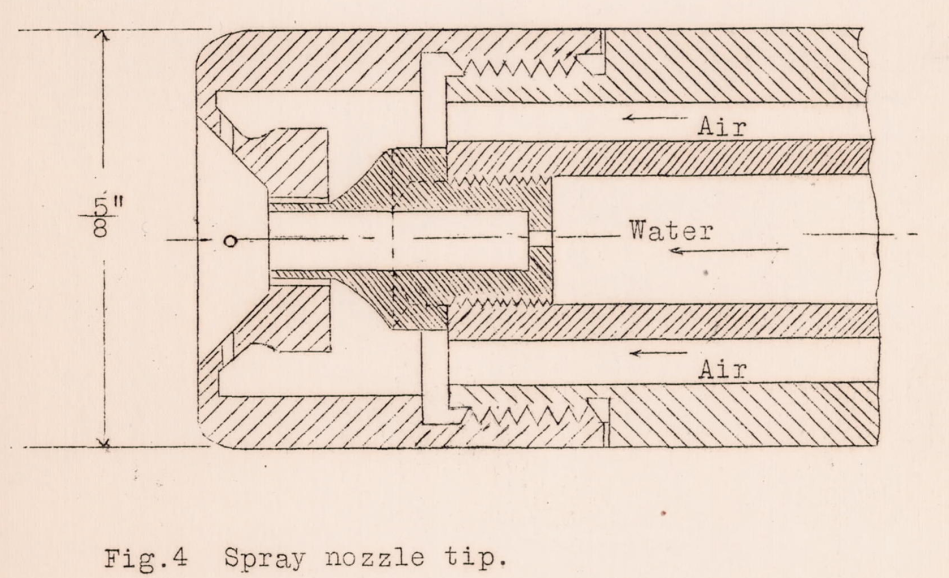
Certain preliminary tests were made in the wind tunnel to determine the best form of water spray necessary to produce ice of smooth appearance and which would be similar in structure and form to that observed in flight (Reference 12), in order to make a study of possible preventives. It was found that the character of the formations obtained depended to a great degree on the quantity of water admitted to the tunnel per unit of time. A spray which consisted of large drops was found to use excessive quantities of water which could not be properly regulated without giving very poor dispersion in the jet, and which gave formations of ice on the model of a very different shape from any obtained in flight. A spray, which consisted of very small drops, was found to be quite satisfactory and was used in all the following tests, as it could be easily controlled to give even dispersion across the air stream at any desired rate of water output.
It is not detailed how the drop size was measured.
Several candidate ice prevention coatings were tested:
Insoluble compounds:
- Light lubricating mineral oil.
- Heavy lubricating mineral oil.
- Cup grease.
- Vaseline.
- Paraffin.
- Simonize wax.Soluble compounds:
- Glycerine.
- Glycerine and calcium chloride.
- Molasses and calcium chloride.
- Hardened sugar solution.
- Hardened glucose solution.In all the tests only half of the airfoil was covered with the substance under consideration, the other half being left bare for comparison. In all accompanying photographs of ice formations, the coated half of the wing is indicated by an arrow.
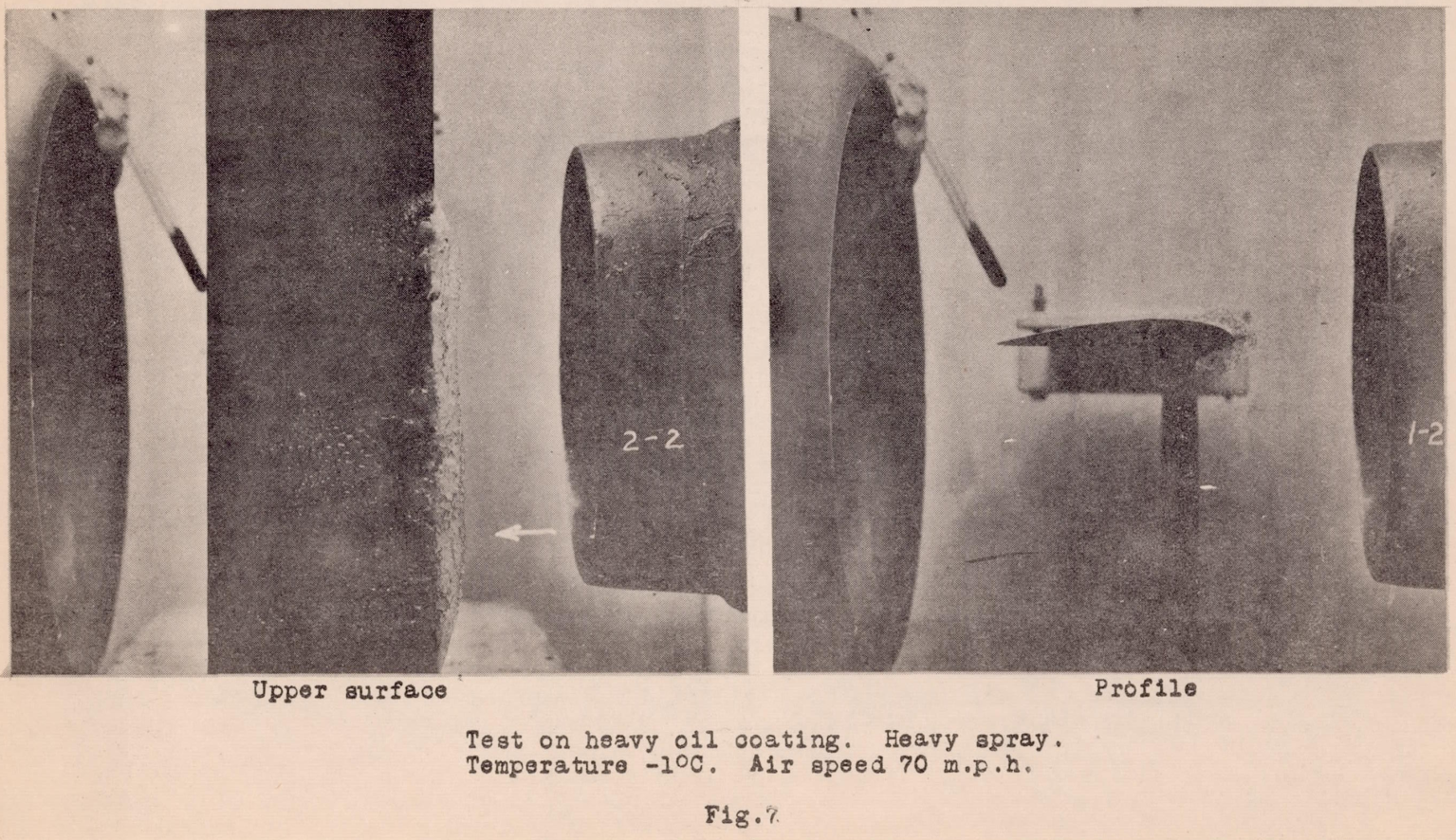
In these first tests, oils, greases, and waxes showed no tendency towards prevention or even hindrance of the formation of ice, and actually, in some cases they augmented it as shown in Figure 7. It was originally believed that the drops would not remain on these substances, since they are supposed to shed water, but this action did not take place. The drops adhered to the surface, especially at the stagnation point of the leading edge, and froze quite as readily as on the bare wing.
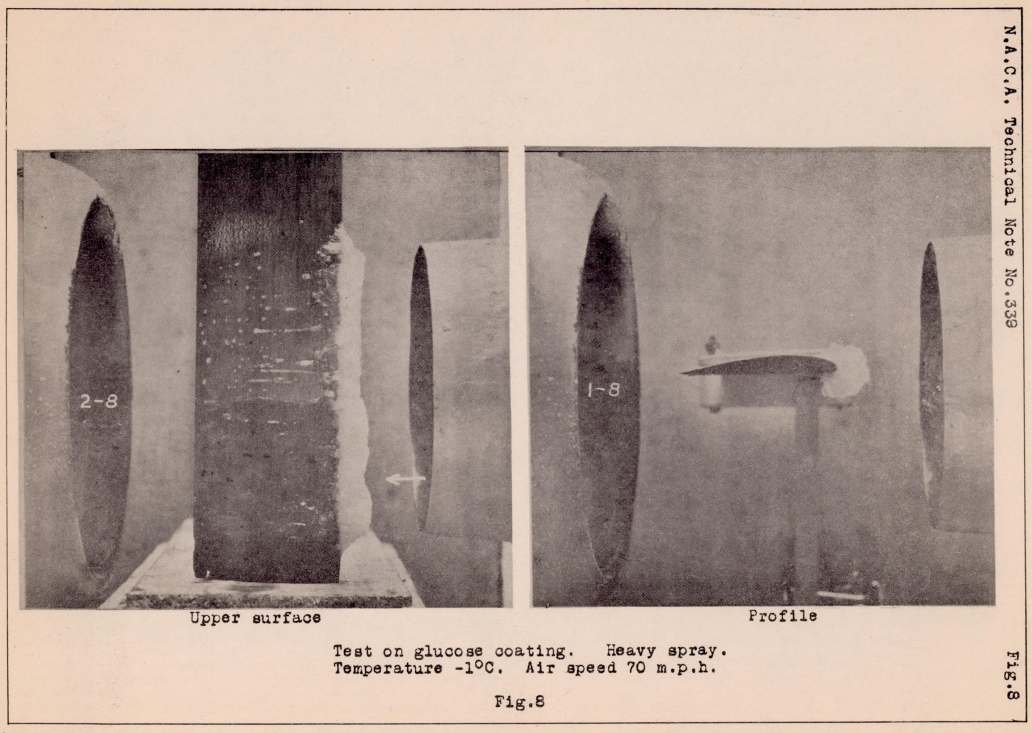
Tests on the soluble substances likewise did not show a marked preventive tendency. It was believed that these substances would dissolve with the water as fast as it struck the surface , and thus lower the freezing point so that ice would not form. Owing to the low viscosity of the glycerine and calcium chloride solutions, they immediately blew back from and exposed the leading edge on which ice then formed. Tests made on the sugar solution did not show this tendency to blow back. The solution, however,was not easily dissolved, consequently ice formed readily on the leading edge on top of the coating. It was difficult to distribute this sticky substance evenly on the wing and some of it crystallized upon cooling, showing that it was a rather impractical coating at the best. In tests on a hardened glucose solution (Fig. 8), made at the suggestion of the U.S. Bureau of Standards, the same general process took place as in the case of the sugar, except that for the first two minutes of the test the formation of ice on the coated side was hindered. However, after the formation had once started, it built up rapidly showing apparently that there was too much water falling on the surface for the glucose to have much effect.
Assuming that the conditions under which the first series of tests was made were too severe and probably not representative of flight conditions, a second series of tests was made which gave less exaggerated formations. These second test conditions were obtained by reducing the quantity of spray water admitted to the tunnel per unit of time to one-fifth of the previous anount, and by reducing the duration of the tests to nine minutes. A few preliminary tests under these altered conditions appear ed to indicate that the formations obtained were more moderate and approximated more nearly those of full scale.
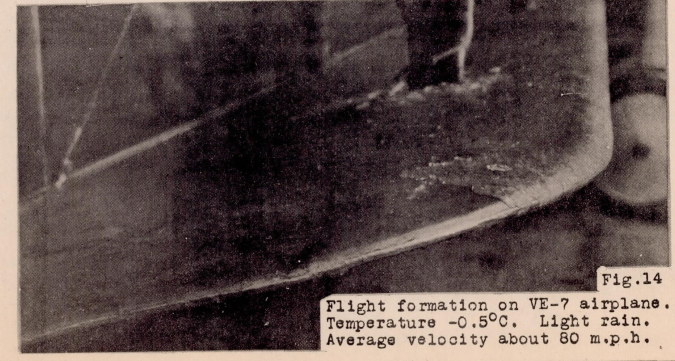
Information on various flight tests has been carefully studied and compared with the wind tunnel results on the uncoated airfoil. For comparison with wind tunnel formations, a photograph of an actual ice formation obtained in a test flight at this laboratory is given in Figure 14. This particular example was obtained during a sleet and rain storm at a recorded air temperature of about -0.5 C. The formation was collected in about 15 minutes, and though very light, had a characteristic shape and structure.
The amount of water present in the wind tunnel jet was measured roughly to determine the actual density of the spray used in the tests. These measurements were compared with meteorological tables, and indicate that the spray density used in the first series of tests, which was about 2000 milligrams of liquid water per cubic meter of air, corresponds to excessive rain. Similarly, the density used in the second series, which was about 500 milligrams per cubic meter, corresponds with figures for a moderate to heavy rain. It is believed that the latter density represents more closely average ice-forming weather conditions.
It is not explained further how the "amount of water present in the wind tunnel jet was measured".
Conclusions
Within the scope of these tests which consisted only in investigating the ability of certain substances when applied to an airfoil surface to prevent the formation of ice, none of the compounds showed any appreciable preventive action with the heavy spray.
The following conclusions may be drawn from the results of tests in which the lighter spray was used:
1. Insoluble compounds, such as oils, waxes, greases, and paints, are ineffective in preventing the formation of ice.
2. Soft soluble compounds, such as mixtures of molasses and glycerine with calcium chloride, which do not have a strong tendency to keep their shape and to adhere to the surface, blow away from the leading edge and do not prevent the growth of ice.
3. Hard soluble compounds, such as glucose and Karo syrup, which hold their shape and adhere strongly to the surface, prevent ice formations within certain temperature limits, depending on their solubility. 4. Coatings need only be applied to the leading edge of an airfoil as far back as the maximum ordinate.
5. Other methods of applying substances to the wing surfaces may prove to be effective, such as the continuous application of a liquid soluble compound to the leading edge.
It is not clear that this particular icing wind tunnel was used for other icing tests. See "We Freeze to Please": A History of NASA's Icing Research Tunnel and the Quest for Flight Safety for more information.
Icing wind tunnel tests lessons learned
NACA-TN-339 illustrates some general "lessons learned" I have from icing wind tunnel tests.
Calibration
NACA-TN-339 had the goal
"to produce ice of smooth appearance and which would be
similar in structure and form to that observed in flight"
Water spray parameters were adjusted to achieve this. Current calibrations typically have MVD and LWC values measured, but in 1930 there were limited means to achieve that.
Sometimes all one needs is a relative result, such as compound A performed better than compound B, where perhaps almost any icing condition would do, but I have found that the time invested in ensuring calibration to your target environment is time well spent.
Spray uniformity
"A spray which consisted of large drops was found to use excessive
quantities of water which could not be properly regulated without
giving very poor dispersion in the jet, and which gave formations
of ice on the model of a very different shape from any
obtained in flight."
Current icing wind tunnel calibration reports usually include a spray uniformity map, usually determined by placing a metal grid in the test section and measuring ice thickness at several locations. Being within +/-20% of center-line or nominal concentration is often considered to be "uniform enough". For large water drop icing conditions, the uniform area may be a rather small portion of the test section.
Different test purposes may have different uniformity requirements.
Iteration
"Assuming that the conditions under which the first series
of tests was made were too severe and probably not representative
of flight conditions, a second series of tests was made
which gave less exaggerated formations."
It is likely that you will learn something during the test, that will lead you to adjust later test conditions.
An exception to this may be qualification or certification tests, where the conditions are prescribed, and (hopefully) one is just confirming the expected result.
Comparison to other test environments
"none of the compounds showed any appreciable preventive action
with the heavy spray."
While the surface coatings selected for test may have been "common sense" based on experience with static conditions, things are different in the dynamic formation of ice in an icing wind tunnel, or natural icing flight conditions.
Many times I have heard variations on the phrase "But it worked in our lab!"
Consider small scale tests
Limited scale icing wind tunnels have similar roles today, testing small components, investigating "icephobics", and research studies.
There are several advantages to the small scale test facilities, including:
- low cost test article construction, low operating and ownership costs
- good access and visibility (the entire test section wall is glass or clear plastic, and removable)
- good test productivity (get to target conditions quickly, easy to change or adjust the test article, no large hoist required)
So, if you have a test that can be scaled down, consider using a smaller test facility.
You might perform a preliminary test at a smaller scale facility, learn some things, and then test at a larger scale facility.
However, there are challenges, particularly if one wishes to scale results up to a larger scale (without larger scale tests). We will cover some of those in a later post in the Icing Wind Tunnel Test thread.
Citations
NACA-TN-339 cites 12 publications:
- Simpson, G. C.: The Water in the Atmosphere. Nature, April 14, 1923, London.
- McAdie, A.: The Principles of Aerography. Rand, McNally & Co., 1917.
- Milham, W. I.: Meteorology. MacMillan Company, 1918.
- Geddes, A. E. M.: Meteorology. B1ackie and Son, 1921.
- Gregg, W. R.: Aeronautical Meteorology. Ronald Press Company, 1928
- Barnes, H. T.: Ice Engineering. Renouf Publishing Company, 1928.
- Kopp, W.: Danger of Ice Formations on Airplane. N. A. C. A. Technical Memorandum No. 499, 1929. NACA-TM-499 ntrs.nasa.gov
- Carroll, Thomas, and McAvoy, William H.: The Formation of Ice upon Airplanes in Flight. NACA-TN-313, 1929. ntrs.nasa.gov
- McAdie, A.: Cloud Formations as Hazards in Aviation. McAdie, 1929.
- Hubner, E.: Ice Formation on Airplanes. German Meteorological Service Report No. 25.
- Barnes, H. T.: Adhesion of Ice Research. National Research Council, 1929.
- Carroll, Thomas, and McAvoy, William H.: The Formation of Ice upon Airplanes in Flight. NACA-TN-313, 1929. ntrs.nasa.gov
NACA-TN-339 is cited once in the NACA Icing Publications Database 2
- Theodorsen, Theodore, and Clay, William C.: Ice Prevention on Aircraft by means of Engine Exhaust Heat and Technical Study of Heat Transmission from a Clark Y Airfoil. NACA-TR-403, 1931. ntrs.nasa.gov
An online search 3 found 9 citations of NACA-TN-339.
Notes
-
Knight, Montgomery, and Clay, William C.: Refrigerated Wind Tunnel Tests on Surface Coatings for Preventing Ice Formation. NACA-TN-339, 1930 ntrs.nasa.gov. ↩