"Although supercooled water has been observed and reported for over two centuries, considerable disagreement exists as to the degree of supercooling possible and the factors that influence supercooling."
"Photomicrographic Investigation of Spontaneous Freezing Temperatures of Supercooled Water Droplets" 1
Abstract
A photomicrographic technique for investigating supercooled water droplets has been devised and used to determine the spontaneous freezing temperatures of supercooled. water droplets of the size ordinarily found. in the atmosphere. The freezing temperatures of 4527 droplets ranging from 8.75 to 1000 microns in diameter supported on a platinum surface and 571 droplets supported on copper were obtained.
The average spontaneous freezing temperature decreased with decrease in the size of the droplets. The effect of size on the spontaneous freezing temperature was particularly marked below 60 microns. Frequency-distribution curves of the spontaneous freezing temperatures observed for droplets of a given size were obtained. Although no droplet froze at a temperature above 20° F, all droplets melted at 32° F. Results obtained with a copper support did not differ essentially from those obtained with a platinum surface.
Discussion
This is the first in a series of NACA studies on the properties of supercooled water. Previous publications were reviewed, a new instrument developed, and spontaneous freezing temperatures determined as a function of drop size.
INTRODUCTION Although many important advances have been made in the control of ice formation on aircraft in flight, little progress has been made toward an understanding of the fundamental processes involved in the formation of ice or the prediction of such formation.
Because the presence of supercooled water in the atmosphere is primarily responsible for aircraft icing, it is necessary to know the properties of supercooled water for a complete understanding of the icing process. Although supercooled water has been observed and reported for over two centuries, considerable disagreement exists as to the degree of supercooling possible and the factors that influence supercooling.
During the last 20 years, a few systematic laboratory investigations of supercooled water have been conducted. The studies of Meyer and Pfaff (reference 1), Tammann and Bickner (reference 2), and Rau (presented in reference 3) in Germany, Doucet (reference 4) in France, Cwilong (reference 5) in England, and Dorsey (references 6 and 7) and Heverly (reference 8) in the United States are of particular interest. Three of the many factors investigated that are thought to influence the degree of supercooling may be particularly important in the prediction of aircraft icing in a supercooled cloud:
1. Effect of contaminating agents, such as undissolved. substances in the water
2. Quantity of water comprising the sample, for example, the diameter of a supercooled droplet
3. Length of time the droplet has been at or below 32° F
The results reported in references 1 and 2 on bulk water indicate that impurities in the water are an important factor In its supercoolabilityThe purpose of an investigation conducted at the NACA Lewis laboratory, and described herein was to study the spontaneous freezing temperatures of droplets within the size range encountered in a supercooled icing cloud in order to determine size dependency. Because the spontaneous freezing temperatures of various samples of water investigated by Dorsey (reference 7) differed by as much as 29° F, although all samples were approximately the same volume, a statistical study of water droplets was made to determine If similar variations in freezing temperature exist for droplets of a fixed size. No attempt was made to find any absolute minimum temperature limit for the existence of supercooled water or to determine the effect of time on the spontaneous freezing temperature. Although further research under conditions closely duplicating the supercooled cloud in the atmosphere is necessary, it is believed that the effect of size and presence of impurities on the state of the water droplets in a natural cloud may be similar to that found in this investigation.
APPARATUS AND PROCEDURE
The apparatus employed for this investigation is schematically shown in figure 1. The droplet-supporting surface (fig. 1(b)) consisted of very thin (0.003-in.) platinum foil 0.180 inch in diameter. Sandwiched between the foil and a temperature equalizing plate of 1/32-inch sheet copper was an iron-constantan thermocouple (No. 28 wire) in excellent thermal contact with the platinum. The copper was soldered to the top of a small cylindrical piece of steel that was heated by induction from a surrounding copper coil. The steel was attached on its lower side to a copper rod that was immersed in a cold bath consisting of dry ice and the solvent Varsol. Radio-frequency heating was used to compensate for the heat sink. The temperature could be rapidly changed or kept nearly constant by varying the power supplied to the radio-frequency oscillator. The platinum support surface was surrounded by a small brass chamber with plastic observation ports. This chamber provided a local atmosphere that was isolated from the exterior atmosphere...
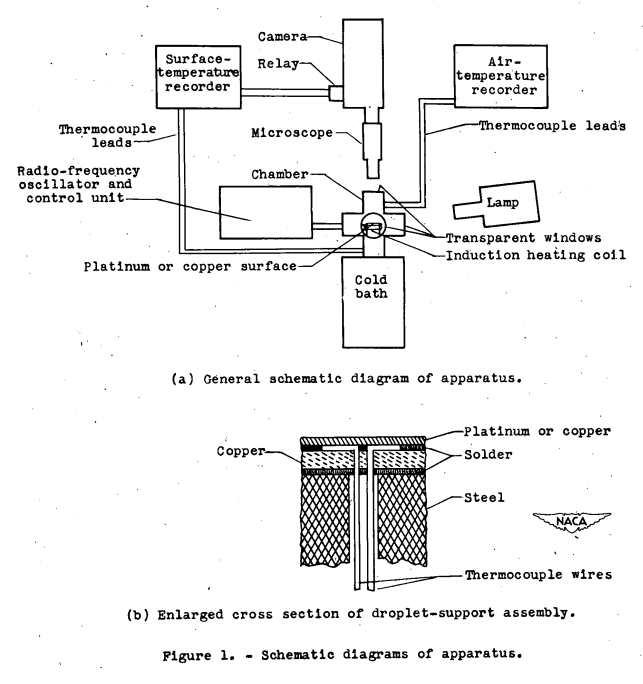
The platinum surface was presumably selected to have a low potential for chemically interacting with the water drops.
A microscope lamp at a 20° angle with the surface was used for illumination. The windows were kept free of condensation by a very light jet of air directed against their external side. A general view of the apparatus with the low-magnification chamber in place is shown in figure 2.
The water used for the drops was obtained through deposition (frost), so it was presumably rather pure, with the possible exception of dust particles ("it is believed that the effect of size and presence of impurities on the state of the water droplets in a natural cloud may be similar to that found in this investigation").
Water droplets were obtained on the surface and then supercooled by the following procedure: Frost was deposited on the surface by lowering the temperature to approximately -30° F; the frost was then melted. Because of surface tension, the water gathered into small approximately hemispherical droplets. The average size of the group of droplets obtained depended on the amount of frost gathered. Next, the temperature was lowered to 32° F and then decreased at a rate of 0.2° to 0.5° F per second.
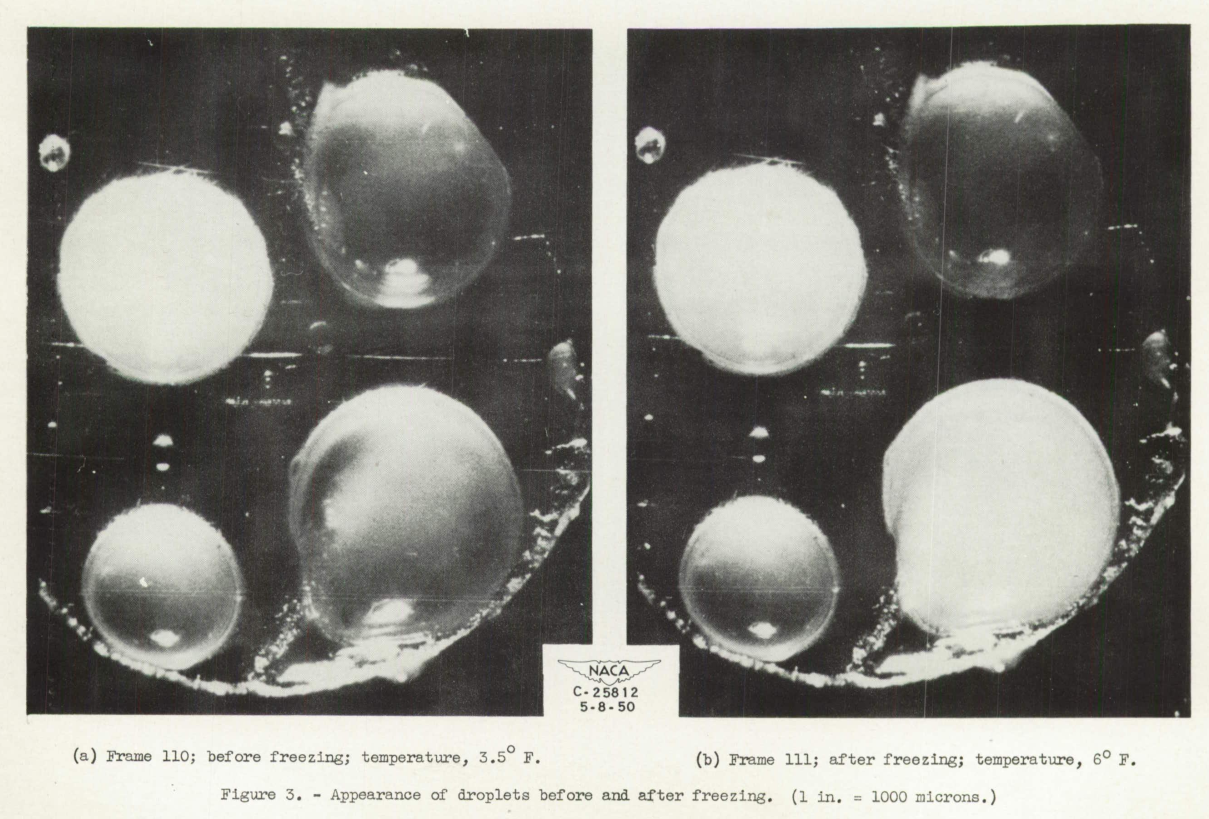
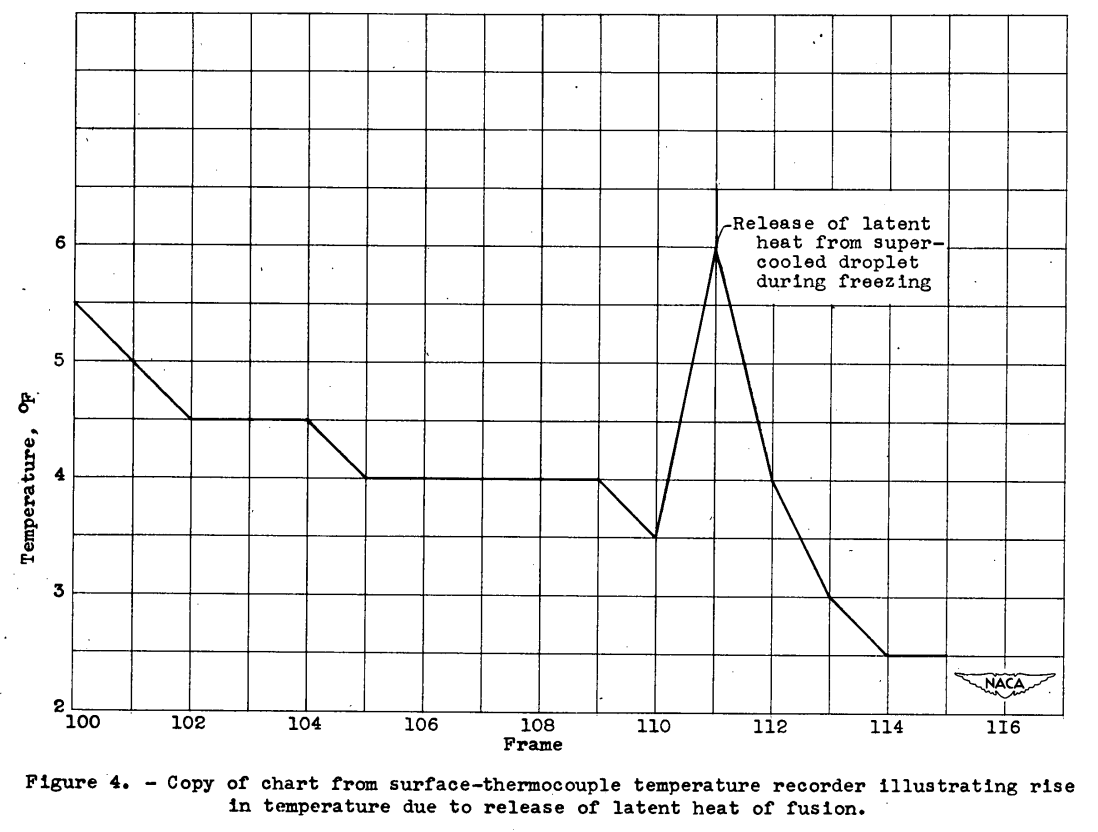
The spontaneous freezing of the droplets was determined by a change in appearance on freezing. In studying each droplet in the photomicrograph frame-by-frame throughout its cooling history, a frame was reached In which a sudden change in opacity occurred. The corresponding temperature was taken as the freezing temperature. As a check on the reliability of this method, a comparison was made between the freezing temperature of large droplets (over 500 microns) obtained by observing the abrupt temperature rise associated with the release of latent heat, and the visual method. In all cases investigated, the temperature at which the abrupt temperature rise occurred corresponded to the freezing temperature as determined from the frame number of the photograph showing a change in opacity. For droplets smaller than 500 microns, the latent heat released could not be detected by the thermocouple in most cases. A very large drop (1600 microns) is shown before and after freezing in figures 3(a) and 3(b), respectively. In the 1-second interval between the two pictures (frames 110 and 111), the large drop in the lower right quadrant of the supporting surface turned opaque or milky.

Figure 4 is a copy of the strip chart from the recording potentiometer. A temperature rise of approximately 2.5° F occurred between frames 110 and 111 due to the release of latent heat as this large droplet froze.
RESULTS AND DISCUSSION
The spontaneous freezing of 5098 droplets was observed. The size, the length of time below the melting point, and the spontaneous freezing temperature of each droplet were recorded. The distribution of droplet sizes for which the spontaneous freezing temperatures were determined is shown in figure 6.
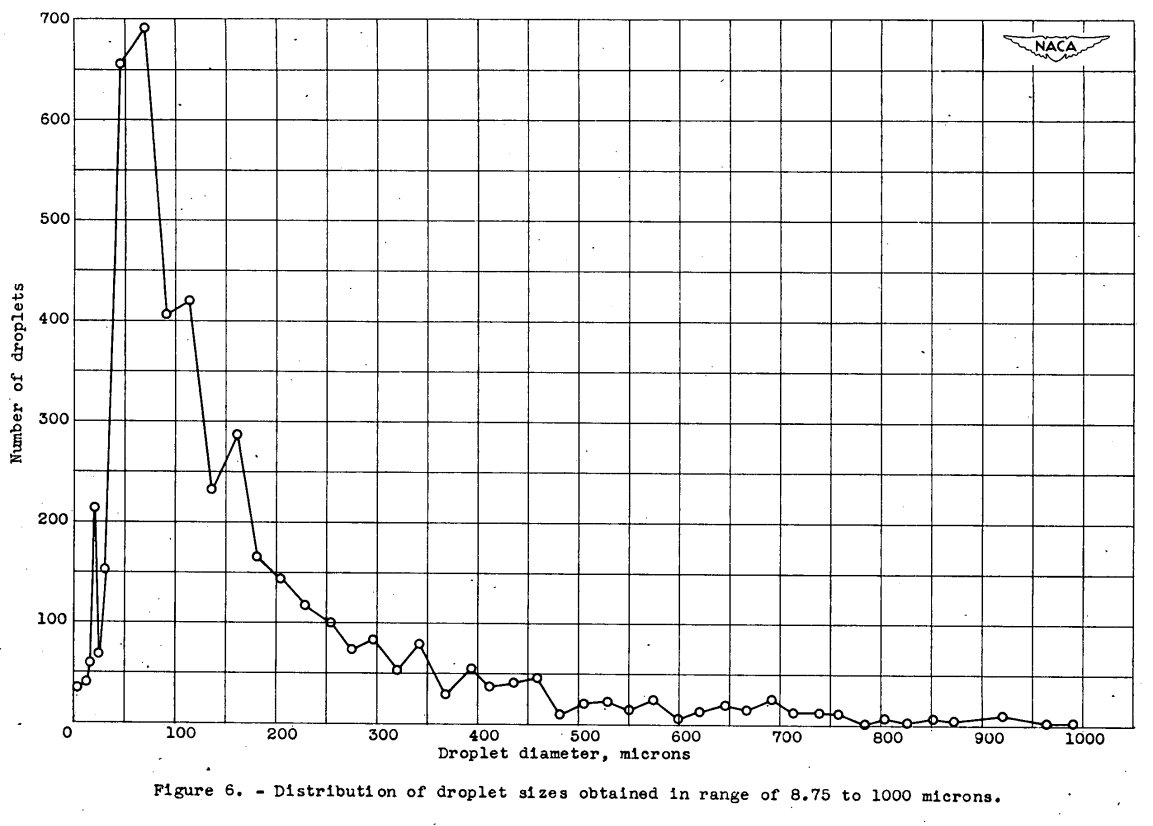
For comparison, a distribution of the mean effective droplet diameters found in icing clouds (references 9 to 11) is shown in figure 7.
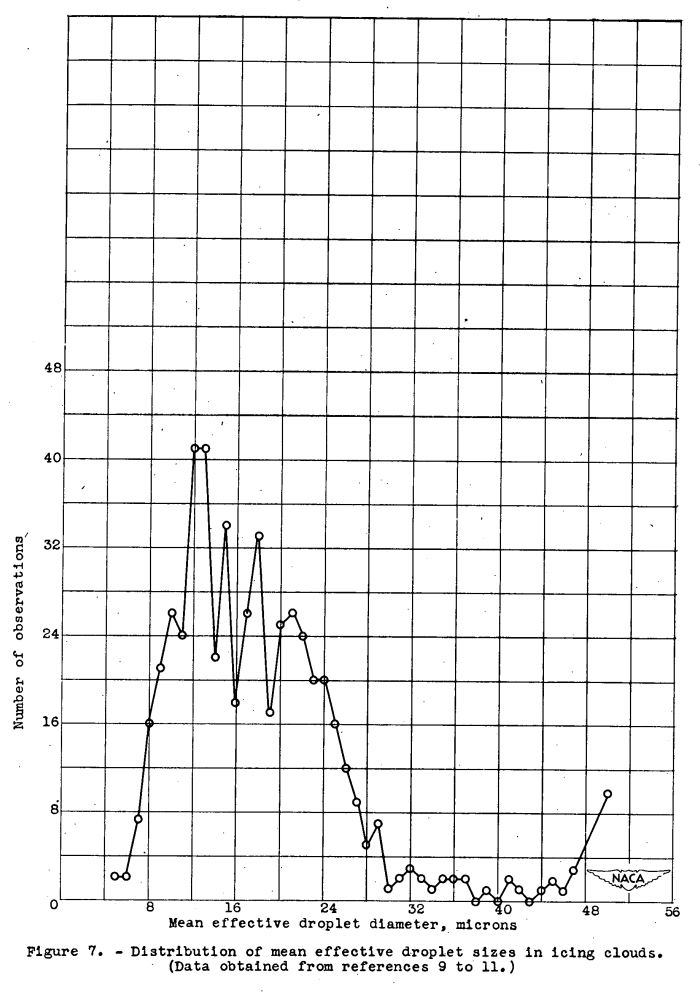
The region investigated in this report corresponds fairly well to the droplet sizes ordinarily found in natural clouds when it is considered that spherical droplets of the same volume as hemispheres would have smaller diameters than that given for the near-hemispherical droplets studied in this report and that the mean effective droplet diameter is a volume-median droplet size.
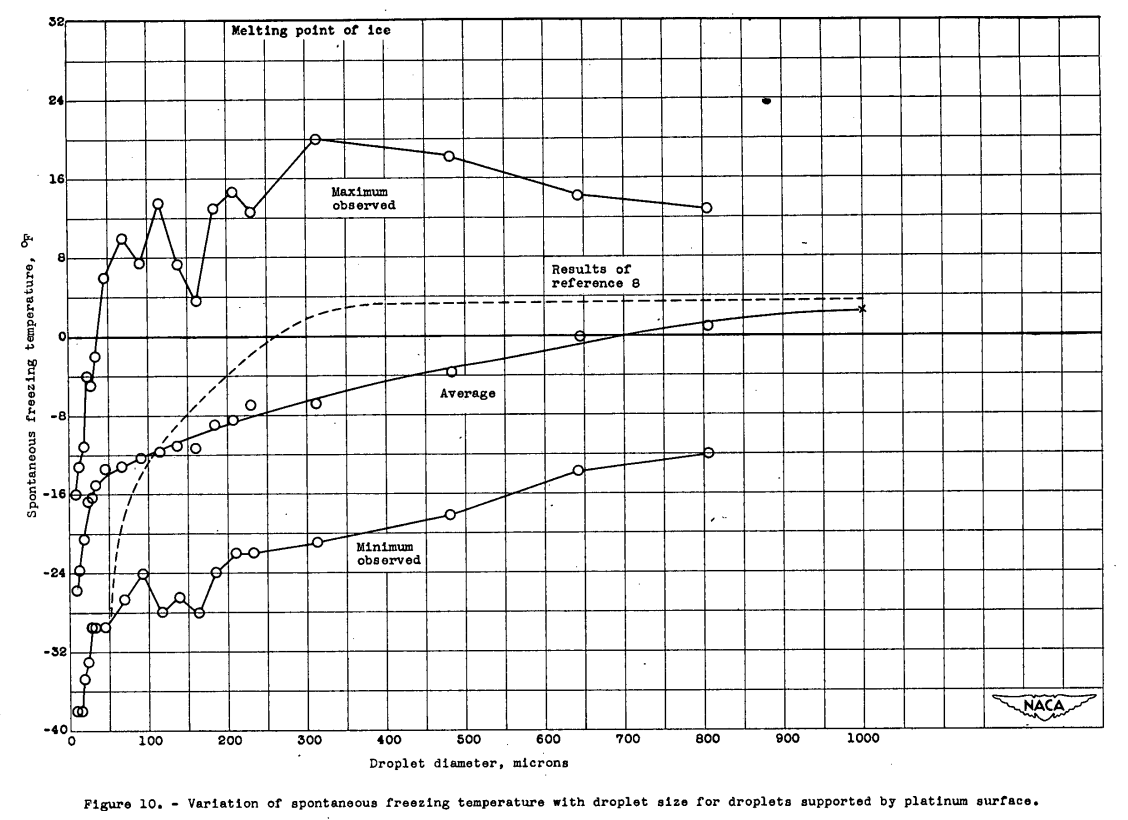
The variation of average, maximum, and minimum freezing temperatures with droplet size taken from the data used for figure 8, with additional data on droplets up to 1000 microns, are shown in figure 10. The average spontaneous freezing temperature for each size decreased as the droplet size decreased for the entire size range. Below 60 microns, the spontaneous freezing temperature decreased rapidly with size. This curve shows the same trend as that published by Heverly (reference 8). The pronounced change in slope in Heverly's curve at 400 microns appears at 60 microns in the data of this report. The wide variation in spontaneous freezing temperature for droplets of a given size is indicated, by the large separation of the curves for the maximum and. minimum temperatures observed for each size. None of the 4527 droplets supported by the platinum surface was observed to freeze spontaneously at a temperature above 20° or below -38° F, but all droplets melted at 32° F.
Conclusions
SUMMARY OF RESULTS
The spontaneous freezing temperatures of 4527 droplets on a platinum surface and 571 droplets on a copper surface in the range 8.75 to 1000 microns were observed and the following results were obtained.:
1. The average spontaneous freezing temperature for each size decreased. as the droplet size decreased, for the entire range investigated. Below 60 microns, the decrease in the spontaneous freezing temperature with decrease in droplet size was particularly marked.
2. The frequency of occurrence as a function of the freezing temperature of droplets of a given size provided a distribution curve with a well-defined peak frequency.
3. The spontaneous freezing temperature of a given droplet tended to be the same on successive freezings.
4. No droplet froze spontaneously at a temperature above 22° F, but all droplets melted at 32° F.
5. Spontaneous freezing data taken with a copper supporting surface were not significantly different from that taken with a platinum support.
Citations
This publication is cited by 47 publications, per scholar.google.com, including some recent publications.
Related
This is part of the Properties of Water thread.
The results from this publication are used in the next publication in this thread, NACA-TN-2234.
"References 9 to 11" are reviewed in the Meteorology of Icing Clouds thread:
Notes
-
Dorsch, Robert G., and Hacker, Paul T.: Photomicrographic Investigation of Spontaneous Freezing Temperatures of Supercooled Water Droplets. NACA-TN-2142, 1950. ntrs.nasa.gov ↩