"Alcohol as a means of protection against ice formation on propeller blades is widely used by commercial air lines on transport airplanes." 1

Figure 2 from NACA-RB-4F06.
Summary
Freezing point depressant fluids were used widely in the NACA-era.
Key points
- The use of freezing point depressant fluids were not pioneered by NACA, NACA studied improvements.
- Analysis methods were developed in the NACA-era.
- Freezing point depressant fluids are still used today.
Discussion
This is a technology that was not invented by NACA. NACA studies sought to improve the use of freezing point depressant fluids.
When mixed with water, a freezing point depressant fluids lowers the freezing/melting temperature of the mixture, and enough fluid can lower that temperature below the equilibrium wet surface temperature, preventing or removing ice.
There are many potential freezing point depressant fluids. In the NACA-era, alcohols were the most widely used. Today, for aircraft deicing on the ground, glycols are widely used. (However, we will not detail ground deicing herein.)
We already saw some uses of alcohol as a deicing and ice prevention fluid in Carburetor and Induction Systems.
Here, we will detail a study of improving propeller deicing, and a study of calculations to determine how much fluid is required.
"An Investigation of the Characteristics of a Propeller Alcohol Feed Ring", NACA-RB-4F06 1
By 1941, NACA had airplanes with robust ice protection (see "Engine Exhaust Heat"), and so could do natural icing flight tests, like the ones described here.
INTRODUCTION
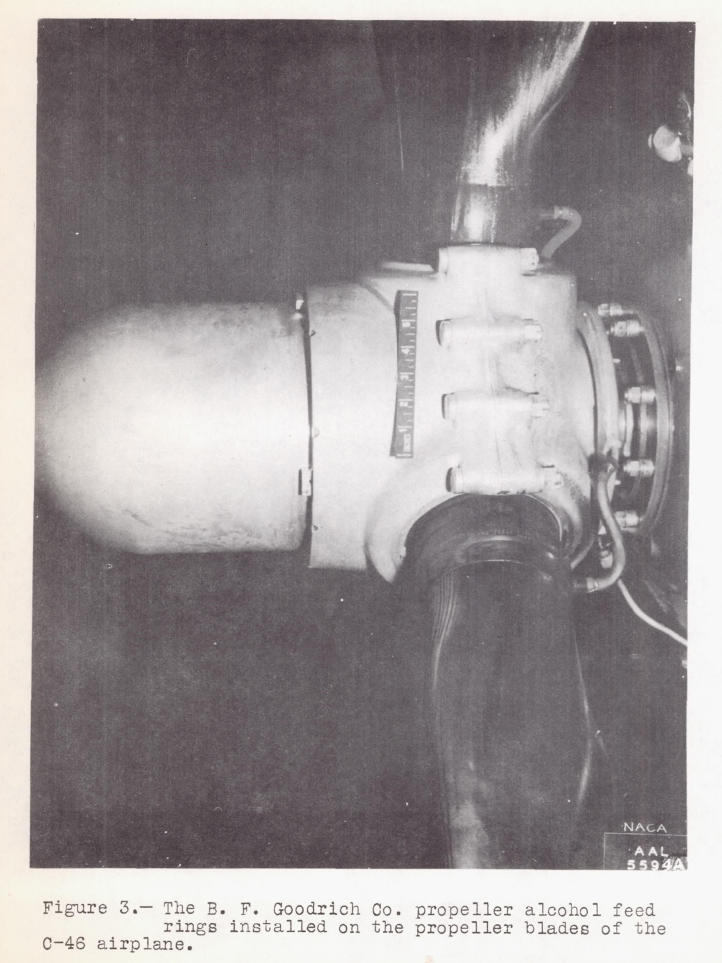
Alcohol as a means of protection against ice formation on propeller blades is widely used by commercial air lines on transport airplanes. The usual propeller alcohol anti-icing system consists of nozzles fixed to the propeller hub which discharge alcohol onto feed shoes mounted on the propeller blades. Delivery of alcohol through the discharge nozzles normally used often results in inadequate protection, since any one location of the discharge nozzle will not provide optimum distribution for all possible propeller-blade angles.
The present investigation was undertaken to determine if improved protection at various blade angles could be obtained through the use of a propeller alcohol feed ring developed by the B. F. Goodrich Rubber Company, Comparative alcohol-distribution tests of one propeller fitted with discharge nozzles and the other propeller fitted with alcohol feed rings were made on a Curtiss-Wright C-46 cargo airplane during flights in clear air. Tests also were made of the effectiveness of the system incorporating feed rings in preventing ice formation during flights in natural icing conditions at the Materiel Command Ice Research Base, Minneapolis, Minn.

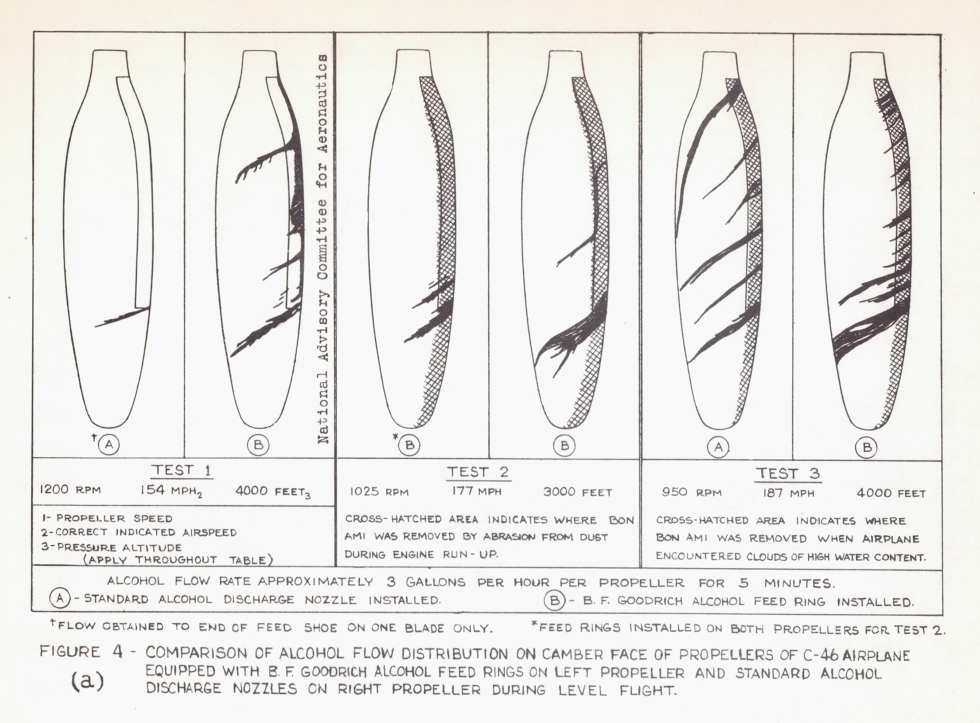
Representative sketches of the flow patterns obtained on the camber face of the propeller blades during these tests are shown in figure 4. There was very little flow over the thrust face of the blades. A flow of alcohol to the end of the propeller feed shoes was obtained with both the feed-ring and discharge-nozzle installations as shown in figure 4, except in test 1 when the alcohol reached the feed-shoe tip only on one blade of the standard nozzle-equipped propeller. The alcohol-flow patterns were partially obliterated during tests 2 and 3 when the coating was removed by unexpected agencies. However, faint traces in the cleared zones and reference to the flow lines outside the cleared areas permitted extension of the flow lines with reasonable accuracy.
The tests of the ice protection afforded by the feed-ring installation were made in conjunction with tests of the thermal ice-prevention equipment installed on the C-46 airplane. The alcohol feed rings were installed on both propellers and the airplane was flown in a wide variety of natural icing conditions. During all icing tests alcohol flow to the propellers was started before entering icing conditions. Protection against loss of airplane performance was obtained in all flights except one without increasing the propeller speed above 950 rpm, which corresponds to 1900 rpm engine speed. The alcohol-flow rate was maintained at approximately 5 gallons per hour per propeller during all the icing flights. Observations with the stroboscope indicated that the formation of ice was not entirely prevented, but that periodic accumulations of ice were satisfactorily removed by the flow of alcohol along the blade leading edges. During one flight in icing conditions at 13,500 feet pressure altitude, propeller ice prutection was not adequate at the cruising propeller speed. Ice formed on the blade leading edges, as evidenced by decreased airspeed and rate of climb. Normal rate of climb and airspeed were restored by increasing the propeller speed to 1200 rpm. which is the ice-emergency propeller-operating speed for the C-46 airplane. After 100 hours of flight service, visual inspection of the feed rings showed no evidence of failure and no maintenance was required.
The comparative alcohol-distribution tests indicated that better blade leading-edge coverage was obtained at all propeller speeds and flight conditions investigated with the feed rings than was possible with the standard alcohol-discharge nozzles. Figure 4 shows that a more efficient use of alcohol was obtained with the alcohol feed-ring installation for all conditions tested. The distribution tests also indicated that alcohol was satisfactorily provided to the blade leading edges at the ice-emergency propeller speed only by the use of the feed rings.
Some engines today have an ice protection procedure that increases the engine speed temporarily, but they are not typically labeled "ice-emergency".
Although the tests in icing conditions indicate that an effort must be made to develop a method of propeller ice protection superior to the alcohol system, the use of feed rings in lieu of the standard discharge nozzle resulted in a definite improvement of the propeller alcohol system. It should be noted that good alcohol distribution and coverage of the propeller-blade leading edge are very difficult to obtain with as large a diameter propeller as were used in the tests and therefore the alcohol system could not have been expected to remove ice accumulations under all conditions, even though an improvement in the system had been made.
CONCLUSIONS
1. The use of the propeller alcohol feed ring resulted in a more satisfactory distribution of alcohol along the leading edge of the propeller blade than did the standard alcohol-discharge nozzles, indicating a greater degree of ice protection and a more efficient use of alcohol.
2. The alcohol feed-ring equipment required no maintenance in 100 hours of flight service.
3. The manufacturer's recommendations for installation of the feed rings were satisfactory.
"Kinetic Temperature of Wet Surfaces A Method of Calculating the Amount of Alcohol Required to Prevent Ice, and the Derivation of the Psychrometric Equation", NACA-ARR-5G13 2
We saw the calculations wet surface temperatures in the review in the Thermodynamics Thread, NACA-ARR-5G13.
Here, we will look at the effect of freezing point depressant fluid, such as ethyl alcohol.
A method is given for calculating the temperature of a surface wetted either by a pure liquid, such as water, or by a mixture, such as alcohol and water. The method is applied to the problem of protecting, by alcohol, propellers and the induction system of the engine against ice. The minimum quantity of alcohol required is calculated for a number of arbitrarily chosen conditions. The effect of evaporation of alcohol is shown repeating the calculations for a nonvolatile fluid. The method can be applied to other problems in evaporation, for instance, to the evaporation of fuel in the induction system of the engine. The psychometric equations, used in wet-bulb hygrometry, is deduced in its general form. The effect of kinetic heating is included.
These calculations are given by way of illustrating the method. Their primary use, it appears, is in calculating the rate of heating required to prevent ice by the method of references 1 and 2. The problem in protection by fluids is to distribute the fluid efficiently. In this case, the method of calculation can be used to find the minimum rate of supply required, or to compare the merits of fluids of different composition, on the assumption of perfect distribution.
Blade Temperatures with Alcohol-Water Mixtures
Calculations have been made for the case in which ice is prevented by supplying ethyl alcohol to the blades of the propeller. The calculations have been repeated for a nonvolatile fluid having the same depressant effect on the freezing point as ethyl alcohol. The difference in the quantity of fluid required gives the excess alcohol which must be supplied in order to neutralize the refrigerating effect caused by its evaporation. A similar calculation has been made for methyl alcohol to show how volatile fluids of different compositions may be compared.
Calculations have been made for a speed of 450 feet per second assuming laminar flow, for blade temperatures of 23 F (-5 C) and also 10.6 F (-12 C), and for barometric pressures of 760 millimeters and 350 millimeters [of mercury]. Two calculations have been made at a speed of 250 feet per second. The results are given in Table II. As an instance of the method, the calculations of the blade temperature of 23 F at 760 millimeters pressure, the first in table II [at an air temperature of 15.2 F], may be cited. For this condition, the concentration of alcohol required to depress the freezing point to 23 F, from the data of the International Critical Tables, is 124 grams per liter of water.
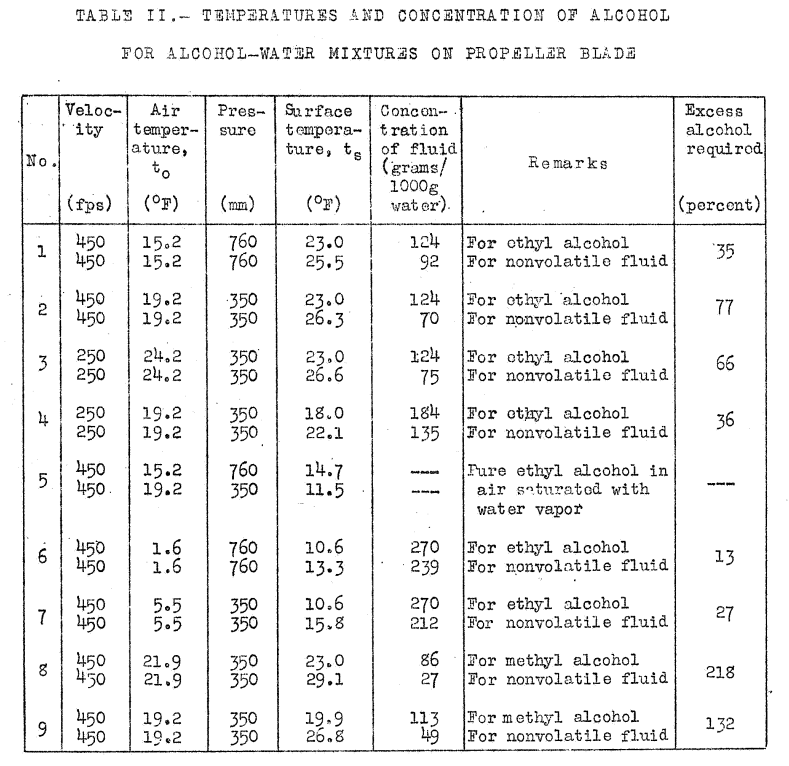
The effect of evaporation is found by calculating the temperature that the blade would assume in air temperature of 15.2 F with a nonvolatile fluid, and calculating the concentration of fluid required. The fluid is assumed to have the same depressant effect on the freezing point as alcohol.
...
the concentration of the [assumed nonvolatile] fluid of the same molecular weight as ethyl alcohol is 92 grams per liter of water. The excess of ethyl alcohol required to neutralize the refrigerating effect produce by its evaporation, therefore, is 35 percent.
See also
- Smith, E. L.: "The Design of Fluid Anti-Icing Systems". Engine Laboratory, National Aeronautical Research Establishment, Ottawa, Canada, Lecture No. 11, University of Michigan Airplane Icing Information Course, 1953. (32 pages) (includes errata sheet)
- Smith, E. L.: "The design of fluid anti-icing systems". National Aeronautical Establishment (Canada) LR-64, 1953. nrc-publications.canada.ca
These provide more design details, and an interesting Figure 15, "Proposed Meteorological Design Requirements for Aircraft Anti-Icing Equipment", which we may detail later in a Meteorology thread or the University of Michigan Airplane Icing Information Course thread.
- Bowden, D.T, et.al., “Engineering Summary of Airframe Icing Technical Data”, FAA Technical Report ADS-4, General Dynamics/Convair, San Diego, California, 1964. apps.dtic.mil
In the thermodynamics thread we previously reviewed ADS-4.
ADS-4 has a section on fluid ice protection systems, and comparisons to other ice protection systems, such as thermal anti-ice.
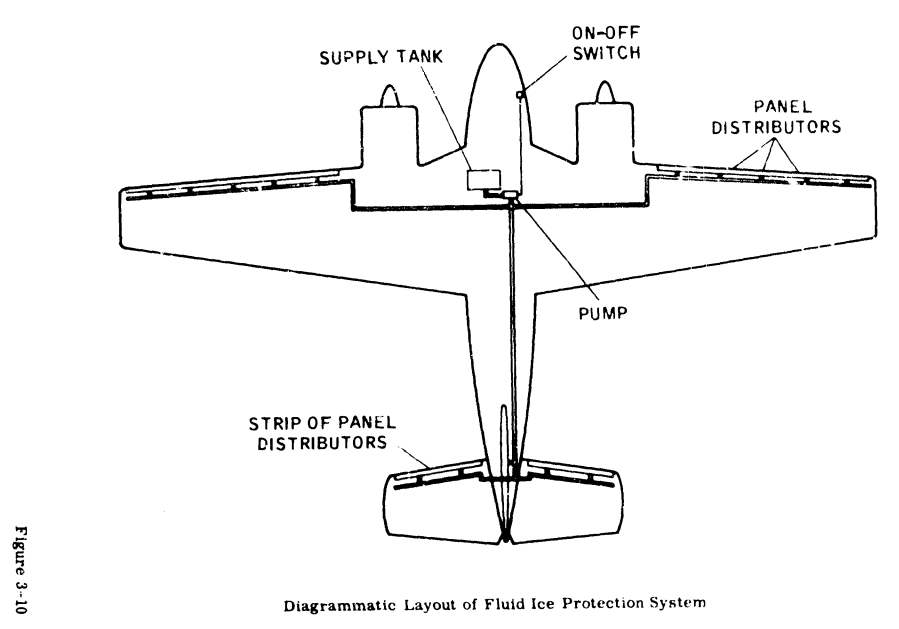
Figure 3-10 from ADS-4.
Conclusions
Freezing point depressant fluids are still used on airplanes today. For the larger jet transports, the use is not common. ADS-4 goes into some of the advantages and disadvantages of fluids versus other protection methods.
Citations
An online search (scholar.google.com) found citations for
NACA-RB-4F06 zero times, and
NACA-ARR-5G13 18 times.
Notes
-
Neel, Carr B: An Investigation of the Characteristics of a Propeller Alcohol Feed Ring. NACA-RB-4F06, NACA-WR-A-50, June, 1941. ntrs.nasa.gov ↩↩
-
Hardy, J. K.: Kinetic Temperature of Wet Surfaces A Method of Calculating the Amount of Alcohol Required to Prevent Ice, and the Derivation of the Psychrometric Equation. NACA-ARR-5G13, 1945. ntrs.nasa.gov ↩